



A new trend among counterfeiters is to target high-value drugs like HIV antivirals in both the developing and developed world. This problem was highlighted in 2020 and 2021, when Janssen Pharmaceuticals and Gilead warned that counterfeits of several HIV medications had been discovered circulating in the US.
In 2022, Gilead sued a ring of drug sellers and distributors that had allegedly sold 85,247 counterfeit vials, worth more than $250 million, to US pharmacies. According to media sources, Gilead’s Biktarvy drug retails for around $3,500 per vial and Descovy for $2,000. Both drugs represent over $10 billion in annual sales for Gilead.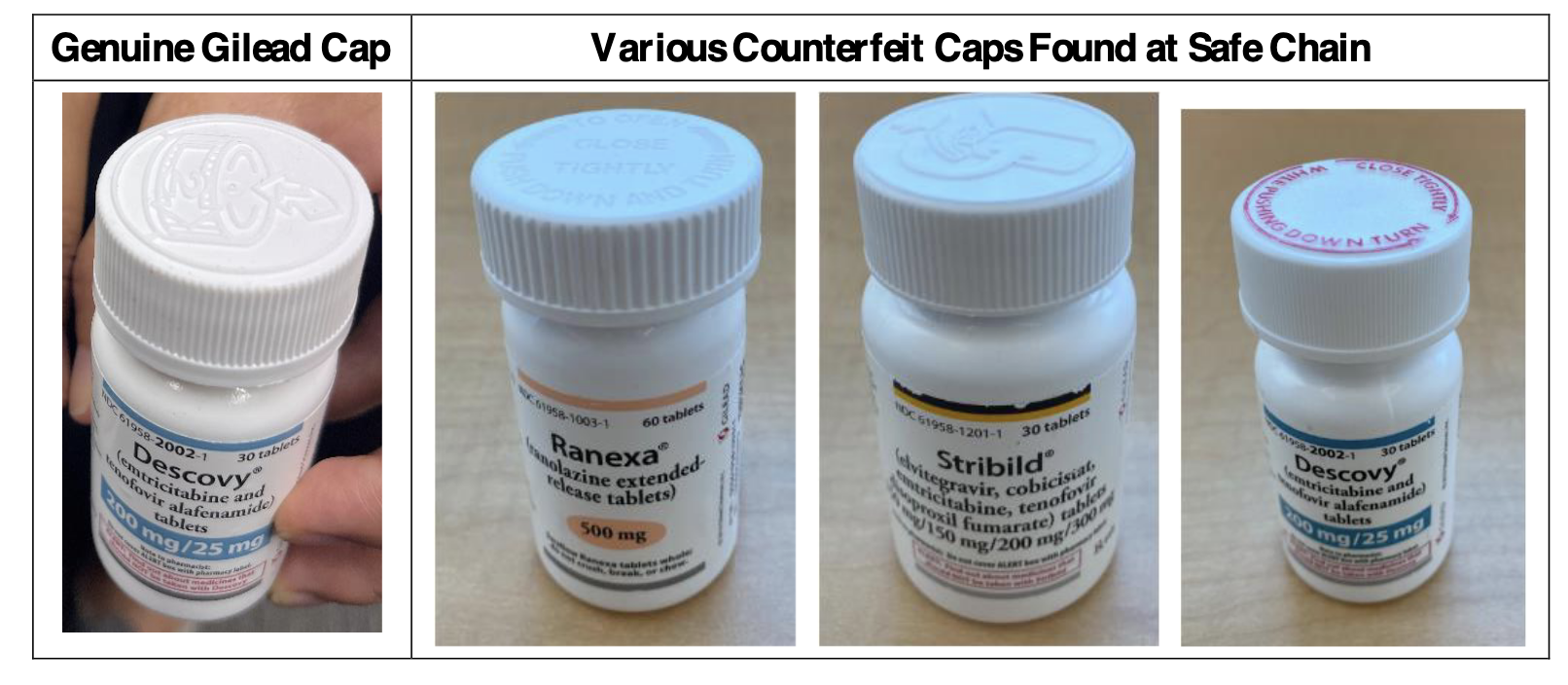
Investigations into the drug ring revealed that unauthorised distributors were selling Gilead drugs to pharmacies sourced from various shell entities, as part of the illegal counterfeiting scheme. The counterfeiters used authentic Gilead vials that, at one point, contained authentic Gilead HIV or other medications. The tamper-evident seals of these authentic vials had been broken, and their contents emptied. The counterfeiters inserted the foreign tablets into these empty vials and then re- sealed them.
The counterfeits were thus engineered to resemble a new, unopened, and authentic vial of authentic Gilead medication. Because federal law requires all prescription drugs to be accompanied by a ‘pedigree’ – a document tracking every sale of the vial from seller to seller, all the way back to the manufacturer – the offenders distributed the counterfeits with falsified documentation fraudulently purporting to trace the counterfeits to an authentic source. This detailed account of the counterfeiters’ modus operandi provides a clear understanding of the complexity and sophistication of their operations.
According to Gilead officials, the company’s actions were instrumental in removing counterfeit HIV medications from the supply chain. Its anticounterfeiting team, headed by Lori Mayall, includes professionals in brand protection, legal issues, supply chains, security, quality, and packaging, who employ measures to detect, stop, deter, and report illicit sales of counterfeit medicines.
From time to time, through its website, Gilead communicates its legal actions and raises consumer alerts and advisories for its consumers. Its global website provides a list of authorised distributors as well as information on how to identify authentic Gilead products.
 Gilead uses comprehensive measures to ensure medicine safety, from tablet design to vials and packaging. Most of its tablets are customised with colour, shape, size, and imprinted codes per FDA guidelines for identification.
Gilead uses comprehensive measures to ensure medicine safety, from tablet design to vials and packaging. Most of its tablets are customised with colour, shape, size, and imprinted codes per FDA guidelines for identification.
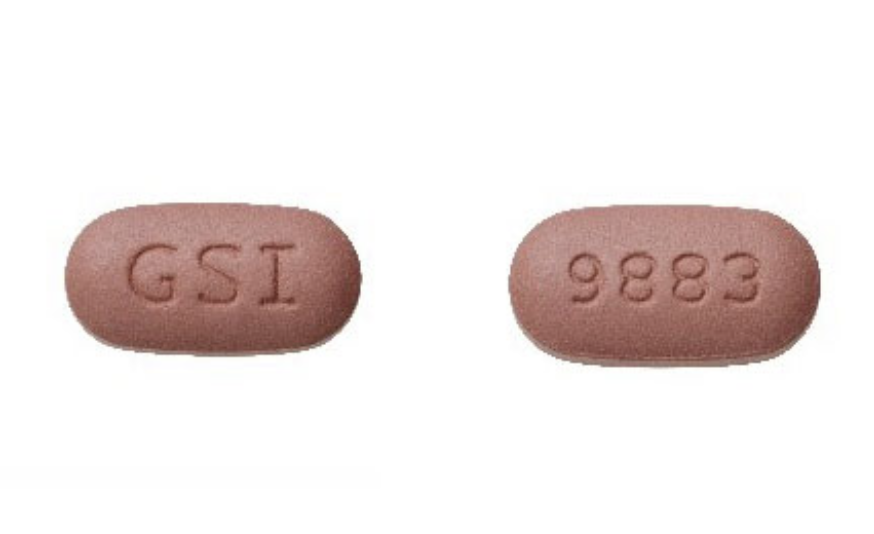
For example, as advised on its website, authentic Biktarvy tablets are purplish brown, capsule-shaped pills with a ‘9883’ code on one side and ‘GSI’ on the other. Similarly, Descovy tablets are blue, rectangular pills with a ‘225’ code on one side and ‘GSI’ on the other. In addition to providing the patient with further identification, these markings make it more difficult for counterfeit versions of the drug to be manufactured. Both brands (Biktarvy and Descovy) are dispensed in white plastic with white caps and Gilead- branded labels.
Gilead’s hugely expensive EPCLUSA® and TRODELVY® medicines in China carry tamper-evident seals on both the top and bottom of their carton packaging, with the top seal comprising a colourshifting hologram and variable QR code.
When the hologram is tilted strongly forward, the image of the small ‘Leaf and Shield’ logo and ‘GILEAD’ name appear bright green against a dark larger logo. When the hologram is rotated 90°, the contrast will flip: the large logo now appears bright green while the small logo and name appear dark.
Both the top and bottom label use VOID technology, which essentially means they are constructed in two ‘parts’. Any attempt to transfer the label to another product will result in the top part being peeled off, leaving behind the irremovable ‘residue’ of the bottom part.
In the case of the Gilead labels, when the top label is removed, the hologram will split into an ‘X’ to indicate the box has been opened, whereas the QR code remains on the box.
In addition, the label can be checked by scanning the QR code with a smartphone, which directs the user to a Gilead response page, notifying them if the QR code is valid.
As for the label on the bottom of the medicine packaging, this has a ‘lock’ VOID effect. When the label is peeled off, two opened lock images appear. This effect is irreversible, ie. the locks remain visible even when trying to reseal the label.
Gilead also uses similar solutions for its pricey Veklury (remdesivir) brand, which treats patients for COVID-19.
With this new trend among counterfeiters to target high-risk, high-cost, life-saving drugs, the need to ensure the authenticity and safety of medicines has become even more crucial for companies like Gilead.
Therefore it is encouraging to see that the company is using a strong combination of physical and digital security features that work together on the same packaging label – as opposed to a standalone 2D barcode offering little in the way of authentication.
https://www.gileadchina.cn/en/anti-counterfeiting/packaging-verification-features-in-china/.